Use the Laws of Thermodynamics to Describe Dna Stability
A series of laws called the laws of thermodynamics describe the properties and processes of energy transfer. Bases Vy n Wy ny n-1 Sugar phosphate backbone.
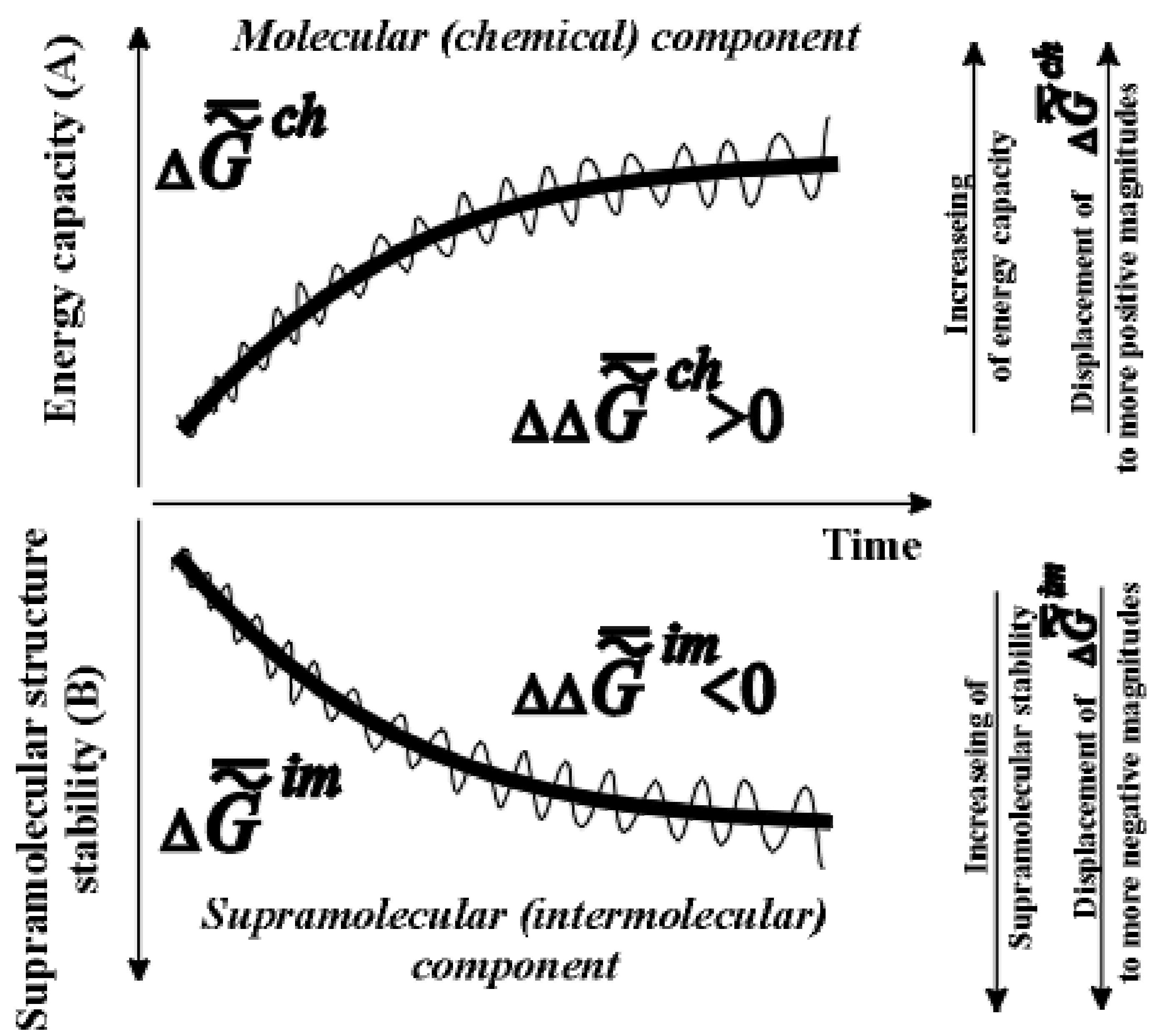
Entropy Free Full Text Thermodynamic Theory Of Biological Evolution And Aging Experimental Confirmation Of Theory Html
The three approaches would be 1 DNA sequencing of this DNA region in all family members with and without the disease to identify differences 2 Compare the amino acid sequence of the orthologous proteins in model organisms to see what is conserved and 3 express the protein from diseased and normal family members and use the biochemical assay in combination with.

. The SECOND LAW OF THERMODYNAMICS states that energy is lost when it is converted from one form to another often in the form of HEAT. The nonequilibrium thermodynamics theory may be another useful approach to describe the physical and biological processes. Thermodynamics investigates systems which can be characterized by state functions.
The thermodynamics of 5-ATGCTGATGC-3 binding to its complementary DNA and RNA strands was determined in sodium phosphate buffer under varying conditions of temperature and salt concentration from isothermal titration calorimetry ITC. This means that energy cant be created or destroyed only transferred or transformed. Tm depends on the length of the DNA molecule and its specific nucleotide sequence.
It is noted that discovery of the law of temporal hierarchies and the substance stability principle made it possible to use quasi-equilibrium thermodynamics to describe aging of organisms and. We have computationally investigated the structure and stability of B-DNA. The Second Law of Thermodynamics.
First however before discussing the Second Law we should define the First Law and for that matter thermodynamics itself. The First Law of Thermodynamics commonly known as the Law of Conservation of Matter states that matterenergy cannot be created nor can it be destroyed. 1to understand the relationship between quantities of heat and work in biological systems.
2to understand the influence of energy changes in biological phenomena. The more spread out the energy the more ways it can exist the greater the entropy of. Thermodynamics has long been a key theory in biology used in problems ranging from the interpretation of binding both in vitro and in vivo to the study of the conformations of DNA whether under the action of optical traps in well-characterized solutions or in the highly compacted state of the cellular interior.
An evolved and adapted biological system converts energy in the efficient manner for transport of substances across a cell membrane the synthesis and assembly of the proteins muscular contraction reproduction and survival. Nucleic acid thermodynamics is the study of how temperature affects the nucleic acid structure of double-stranded DNA. To this end we have analyzed the bonding in a series of 47 stacks consisting of two base pairs in which the base pairs cover the full range of natural Watson-Crick pairs mismatched pairs.
The first and second laws of. ΔE qw Δ E q w. A living cells primary tasks of obtaining transforming and using energy to do work may seem simple.
Here is a reaction diagram that illustrates D GIf the D G is negative meaning the products are at a lower energy than the reactants then the reaction is thermodynamically favorable meaning it can proceedRemember the second law of thermodynamics states that a system will always want to move toward a lower energy state. The Gibbs free energy change Δ G of the DNA hybridization reactions increased by about 6 kJ mol 1 from 20 C to 37 C. It deals with the total amount of energy in the universe and in particular it states that this total amount does not change.
DNA when in a state where its two. Since their conception however these laws have become some of the. A way of expressing the first law of thermodynamics is that any change in the internal energy E of a system is given by the sum of the heat q that flows across its boundaries and the work w done on the system by the surroundings.
The laws of thermodynamics in principle describe the specifics for the transport of heat and work in thermodynamic processes. However the second law of thermodynamics explains why these tasks are harder than they appear. Crocode of which Schrödinger spoke have become the DNA and the genetic code of today.
This law says that there are two kinds of processes heat and work that can lead to. The first law states that the total amount of energy in the universe is constant. Put another way the First Law of Thermodynamics states.
Let us try to use the second law of thermodynamics to describe the. It can change from solid to liquid to gas to plasma and back again but the total amount of matterenergy in the universe remains constant. The second law of thermodynamics is the entropy of an isolated system only increases Entropy is the shape of energy the ways it can be stored.
Explanation of the analogy relating the stability of equilibrium thermodynamical states and the stability of bio-. Epigenetic influence on DNA and genetic apparatus as a whole. To predict the effect of temperature on a variety of.
Origin of life and its evolution are the result of action of laws of hierarchical thermodynamics of complex systems. Thermodynamics is a compound of two Greek words therme heat and dunamis power. The following steps in a thermodynamical scheme it is obligated.
Answer 1 of 7. Thermodynamics is Not Just for Dead Stuff. The quantity of matterenergy remains the same.
The First Law of Thermodynamics. Objectives of thermodynamics All chemical physical and biological processes are ultimately enabled and regulated by the laws of thermodynamics. Structure of the DNA model left Morse potential between bases right Vy n is the mores potential used to describe the behaviour of the hydrogen bonds and Wy n y n-1 is potential modelled to describe interactions of adjacent bases.
Understand how the second law of thermodynamics applies to biological systems. As cells go through transformations they INCREASE the total entropy of the universe resulting in an DECREASE in available energy. It is the science that speaks of the power or energy contained in heat and its conversion to other forms of energy.
The first law of thermodynamics thinks big. The origin of life can be explained through the study of thermodynamics of the universes evolution. Stability principle made it possible to use quasi-equilibrium thermodynamics to describe aging.
The melting temperature is defined as the temperature at which half of the DNA strands are in the random coil or single-stranded state.
Pdf What Is The Role Of Thermodynamics On Protein Stability

Comments
Post a Comment